case study |

Photos: Andy Caulfield
|
 |
Acorda Therapeutics Inc. built a series
of five engaging and educational
experiences featuring more than 20
interactive screens to engross physicians and communicate key Ampyra data.
|
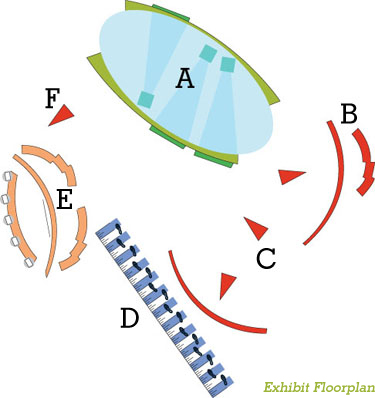
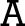 IPAD CIRCLE IPAD CIRCLE
Visitors used 10 iPads to navigate a neuron field. Embedded touchpoints along the journey prompted videos or facts, the appearance of which highlighted how Ampyra may interact with damaged tissue.

 RECEPTION DESKS RECEPTION DESKS
Staff at reception
desks located near booth
entrance points fitted
guests with RFID tags.
 INTERACTIVE KIOSKS INTERACTIVE KIOSKS
Scattered throughout the
display, interactive kiosks
with built-in touchscreens
allowed physicians to sort through Ampyra PI data.

 25-FOOT WALK 25-FOOT WALK
Physicians walked on a set of sequential video footprints down a 25-foot, runway-like monitor. The footprints lit up at different speeds designed to mirror changes in walking speed associated with mild, moderate, and severe MS.

 AMPYRA CHALLENGE AMPYRA CHALLENGE
Physicians used five iPads on cylindrical stands to answer trivia questions about Ampyra. The fastest player to lock in the correct answer advanced his or her avatar along a crosswalk.

 TOUCH WALL TOUCH WALL
Four-foot-square
rear-projection touchscreens tasked visitors with dragging molecules of Ampyra into damaged nerves to stop the leak
of potassium.

|
 |
 t's nearly impossible to avoid the cacophony of pharmaceutical commercials. Just watch prime-time television for an hour or two, and you're likely to see a bevy of pharma ads - many featuring actors skipping through fields of flowers - that all seem to end with a serene voice saying something like "potential side effects include headache, internal bleeding, and nausea." In fact, recent studies estimate the pharmaceutical industry spends between $20 billion and $50 billion a year on marketing, making for a crowded medical marketplace. t's nearly impossible to avoid the cacophony of pharmaceutical commercials. Just watch prime-time television for an hour or two, and you're likely to see a bevy of pharma ads - many featuring actors skipping through fields of flowers - that all seem to end with a serene voice saying something like "potential side effects include headache, internal bleeding, and nausea." In fact, recent studies estimate the pharmaceutical industry spends between $20 billion and $50 billion a year on marketing, making for a crowded medical marketplace.
You can find further evidence of the crowded marketplace at your local store, where shelves are crammed with many of the 100,000-plus over-the-counter drugs and drug combinations registered with the Food and Drug Administration (FDA). Asking your spouse to pick up some Tylenol now involves specifying if you want regular or maximum strength, daytime or nighttime formulations, or one that will also treat a sinus headache or cough.
And the FDA keeps adding to the pile. It approved 21 new drugs in 2010, while also giving the nod to nearly 75 new configurations or uses of existing
medications. For those new drugs, breaking into the consciousness of doctors who will ultimately prescribe them is crucial to
getting a foothold in the market.
But traditional advertising won't necessarily gain the attention of doctors bombarded by persuasive pharmaceutical sales representatives - figures from the Pew Prescription
Project estimate there's one drug rep for every seven physicians in the United States. And mass advertising isn't engaging or interactive enough to sufficiently educate doctors on new drugs, explains Mike Russo, director of corporate digital marketing strategy for Acorda Therapeutics Inc., a biotechnology company with headquarters in Hawthorne, NY.
But one forum is just what the doctor ordered for dishing out education on emerging medications, Russo says, and that's trade shows. "At trade shows, you get a high level of engagement and interaction from attendees. People that have taken the time to travel there are typically there to learn, so you tend to get their relatively undivided attention. You don't get that by simply placing advertisements," he explains.
Educate, Don't Inundate
So in the summer of 2009, when Acorda's marketing team began
planning for the debut of its new drug, Ampyra, its trade show strategy took center stage. Acorda creative director Maria Verastegui faced the daunting task of developing an exhibit concept to facilitate physician education on the drug, which is indicated to improve walking speeds in patients with multiple sclerosis (MS), a disease that damages nerve cells in the brain and spine, impairing nerve communication and resulting in
physical and mental disabilities.
Studies by the National Multiple Sclerosis Society have shown two-thirds of MS sufferers report difficulty walking, and 70 percent report that those difficulties represent the most challenging aspect of living with the disease. While Ampyra hadn't yet attained FDA approval, buzz in the industry and medical community was strong, as Ampyra would be the first drug to actually treat a symptom of MS instead of simply slowing the inevitable progression of the disease.
The Acorda team tentatively planned Ampyra's trade show unveiling for the 62nd annual American Academy of Neurology (AAN)
conference in April 2010. "This would be our chance to put our first foot forward into the market for Ampyra, which had been in development for around 15 years," Verastegui says.
And getting that first step just right for a new drug is high stakes in the pharma industry. While Acorda hasn't released an official figure yet, industry associations estimate companies spend upwards of $1 billion to take a drug from research and development to FDA approval. With such a huge investment of time and money, even the smallest misstep in introducing Ampyra wasn't an option.
For Verastegui and her team, a sure-footed product launch for Ampyra hinged on doctor education. "We had to educate doctors not only on what Ampyra is and how it works, but also on why they should be prescribing it and what kinds of positive effects it can have on their MS patients," she says.
"Since we were educating doctors about a new drug, we needed to engage neurologists in a way that would make our content memorable since many of them were not familiar with our product," Russo says. "Research shows us that people tend to be more receptive to content and retain messages when they can interact with it at their own pace. For that reason, we felt an exhibit full of engaging, educational, interactive technology was the logical solution for our display."
Unpleasant Side Effects
But putting together an exciting and interactive exhibit wouldn't be easy. Introducing a new medication comes with some serious headaches. When exhibit planning began in June 2009, the lack of FDA approval meant content for the display was uncertain. "The FDA approves a product information (PI) sheet for new drugs. That is the only information that can be used in marketing," Verastegui says. Thus, without an approved PI, Acorda was unable to begin crafting its exhibit-marketing message.
Furthermore, Acorda's hopes to steal the show at AAN hung in the balance of the FDA approval process. If approval didn't come through before the April 2010 show, the entire exhibit would be a no-go. As the company's key demographic target for outreach on Ampyra is neurologists, AAN presented the best opportunity, and one of the only opportunities, to educate that audience, Russo explains. "If we didn't get approval in time, we'd have had to wait an entire year for this opportunity to come around again," he says.
Anticipating FDA approval would come hazardously close to the show date, Acorda needed a booth that allowed for last-minute information updates. "Because of the uncertain approval timing, we knew we needed the booth to feature digital signage that could be easily updated when the PI was approved, rather than traditional graphics. Otherwise, the physical construction of static informational pieces would have taken too long to build," Russo says. "That really pushed us toward a largely electronic and digital display."
Prescribing the Right Design
Needing an interactive exhibit display heavy on digital technology, Acorda turned to designer Mitchell Mauk, principal of San Francisco-based Mauk Design Inc., to prescribe the perfect design. While Acorda's marketing team had developed a branding scheme for Ampyra, Mauk faced the challenge of bringing it to life via an interactive display. "We needed to integrate the Ampyra brand, including the colors and typography, in a display that met Acorda's objective of education through interaction," Mauk says. "We had to take something that was little more than a two-dimensional marketing concept and bring it to life."
To do so, Mauk's master plan called for five interactive stations to immerse, engage, and educate attendees using product information. Each interactive station featured an activity or interface to communicate
information doctors needed to understand and prescribe Ampyra, including how it works, who it's for, and key safety and risk information. The stations included self-directed modules and competitive challenges to facilitate mixed learning styles. "When designing the display, we took into account the different ways people learn so we could accommodate everyone," Russo says. "We wanted a memorable way to get to our messages across in whatever way attendees learned best."
With an exhibit prescription calling for heavy use of technology, Mauk focused on keeping the display environment slick and simple to avoid electronics overload. "Sometimes cumbersome technology can distract from the overall message and impact of an exhibit, and we wanted to avoid that," Mauk says. "So we designed the exhibit in such a way to disguise the equipment supporting the technology within the exhibit structures. It looked simple and effortless. You never would have known that there was that much technology."
But Mauk and the Acorda creative team didn't work in a design bubble. With heavy scrutiny and regulation around marketing pharmaceuticals, they endured a lengthy back and forth with a review team consisting of Acorda medical, legal, and regulatory experts. "Given the regulations with pharmaceuticals, it's critical to review design plans and ideas with these parties before the money is spent on the actual product build," Verastegui says. After nearly two months of arduous approvals, reviewers gave the go ahead for a final design.
Acorda teamed with Boston-based Access TCA Inc. for exhibit fabrication,
while digital-marketing agency StudioPMG, based in Irvine, CA, provided expertise for installing the technological infrastructure. The latter included more than 20 interactive screens, from iPads to touchscreen kiosks to a large circular sign that spanned the entire circumference of the 40-by-40-foot exhibit. Constructed from lightweight LED pixel tiles, the 3-foot-tall sign was suspended over the display and illuminated Ampyra brand messaging in its trademark orange and green colors.
While FDA approval was still pending, the flexibility of a digital exhibit platform allowed the build to begin before finalization of the PI information for the display. On Jan. 22, 2010, the exhibit team breathed a collective sigh of relief when the FDA officially approved Ampyra, while also sounding the starting shot on a mad dash to wrap up the build and put on the finishing touches before the show opened.
From Waiting Room to Show Floor
To promote Ampyra's debut at the show, Acorda sent a mailer to registered attendees from a listing provided by AAN. "The mailer provided our showroom location and booth number, and invited attendees to play the Ampyra Challenge," Russo says.
The Ampyra Challenge was a reference to one of the five interactive exhibit areas, a multiplayer game at the outer corner of the booth. It consisted of five iPads on cylindrical stands with one 50-inch plasma screen mounted above. To take part in the challenge, each doctor used an iPad to create an avatar to represent him or her in the game against other visiting physicians. The iPads displayed trivia questions on Ampyra, and the player to most quickly lock in the correct answer advanced his or her avatar along a crosswalk on the plasma screen, a not-so-subtle reference to how Ampyra is intended to improve walking abilities in MS sufferers. The fastest player across the street won, with the winning time and player name recorded on a leader board.
While no prize was offered, Acorda felt the competitiveness of an audience full of doctors would garner interest and keep winning players coming back to up their score while simultaneously enhancing their knowledge of Ampyra. "Although we didn't offer a prize for winning, we find that this particular demographic enjoys participating in competition with colleagues. We felt the spirit of friendly competition would help bring visitors to the display," Verastegui says.
And they were right. When the doors opened on the AAN
show, held April 10 - 17 at the Metro Toronto Convention Centre,
attendees
flocked to the display for the chance to compete against their colleagues and to learn more about Ampyra. A team of nine Acorda representatives greeted physicians at reception stations positioned at the main display entrance points. There, they tucked small radio-frequency identification (RFID) tags behind visitors' main conference badges to track traffic and trigger an automated greeting at the first station each doctor visited. "If you made it through two of the five interactive stations, you would have received all of the key messages on Ampyra that we wanted to communicate," Russo says. To encourage attendees to make that journey, Acorda staff working the booth awarded each doctor visiting
two or more stations a CD-ROM with MRI images of patients with MS,
a learning tool doctors commonly
use when giving educational presentations or training other physicians.
Acorda staff circulated
to help direct doctors to each of the interactive areas. As expected, the Ampyra Challenge drew heavy interest. "We would commonly have five physicians playing the game, and an additional 25 or more standing around and cheering them on," Russo says. "It helped generate interest and excitement, pulling people into our booth so they could learn more about our product and company."
Two other areas featured games for noncompetitive learners, and also proved to be big hits. The first, deemed the "touch wall," was a 12-foot-high structure with three built-in, 4-foot-square rear-projection touchscreens. On each
screen, players had to drag molecules of Ampyra
into neurons damaged by MS to stop the leak of potassium. This action
mimicked the way in which Ampyra is believed to work in treating damage from MS. "After a certain number of molecules had been placed into the virtual channels, pop-up bubbles highlighting Ampyra PI data would
appear on the screen," Russo says. "We believe the physicians that interacted with this display had a much greater chance of remembering how our drug is thought to work than if we just spouted out this info in a traditional presentation, because the activity was interactive, tactile, and more dynamic than a dry PowerPoint."
 The second game area featured a round table fitted with 10 iPads. On each, players navigated a neuron field, with touchpoints along the journey highlighting info about the drug. "The iPad interaction was a first-person, 3-D fly-through of a neuron field," Russo says. "Embedded in the 3-D space were points where a fact or video popped up, highlighting where in the neuron field damage may occur due to MS, and show where our drug would potentially interact with that damaged tissue." The second game area featured a round table fitted with 10 iPads. On each, players navigated a neuron field, with touchpoints along the journey highlighting info about the drug. "The iPad interaction was a first-person, 3-D fly-through of a neuron field," Russo says. "Embedded in the 3-D space were points where a fact or video popped up, highlighting where in the neuron field damage may occur due to MS, and show where our drug would potentially interact with that damaged tissue."
For those wanting more facts than activities, four double-sided kiosks positioned throughout the exhibit allowed them to quickly sort through Ampyra PI data. "We broke out the PI on the kiosk touchscreens into easy-to-use categories like Safety
& Efficacy, About Ampyra,
and References, to make it easy
for physicians to get the information they needed," Russo says. "We find that physicians want to take a longer time exploring the content that is of interest to them, and the kiosks facilitate that exploration."
The final and largest of the five interactive areas had perhaps the biggest impact on visitors. Running across the center of the display floor was a timed walk on a 25-foot-long digital monitor. Physicians walked on a set of sequential video footprints that lit up on the runway-like surface at different speeds designed to mirror average changes in walking speed associated with mild, moderate, and severe MS. "Once the interaction was initiated, the physician would pick which level of disability he or she would be following when walking down the runway. Many physicians did all three levels to get a feeling for the differences in mobility," Russo says. The decision to use the walkway also tied back to techniques used in clinical studies for Ampyra. "The way we measured changes in walking speed in our clinical studies of Ampyra was on a timed 25-foot-walk for patients using the drug."
"When your walking speed is slowed down, it has a very troublesome and significant impact on your daily life, and this activity really got that point across," Verastegui says. "It was very eye-opening for physicians to understand what it was like to have your ability to walk even slightly impaired," Russo adds. By showing doctors the significance of even mild walking impairment, it drove home the message that Ampyra could provide a life-changing benefit for MS patients.
Diagnosis: Success
By the time the show ended and the RFID data was analyzed, it was clear Ampyra's 2010 AAN debut was a rousing success. Approximately 10 percent of the 6,500 show attendees
stopped by Acorda's booth and made it through at least two of the interactive stations. Russo says that's a 50-percent increase from Acorda's previous AAN exhibits.
All visitors received a thank-you mailer from Acorda following the event containing additional information about Ampyra and a link to the product website. Visitors who opted in to the company's communication platform received additional contacts, including phone calls from Ampyra sales representatives.
While Russo says Acorda is still wrestling with determining the precise ROI for the $300,000 display, Ampyra sales figures have been astounding. In the 10 months it was available in 2010, 10 percent of the 400,000 MS sufferers in the United States received a prescription for Ampyra. In fact, Ampyra-related net revenue in 2010 was $52.3 million, accounting for 40 percent of Acorda's yearly income. Helping to offset costs, Acorda continues to use the interactive exhibit at industry shows, and used the booth again at AAN in 2011.
"The success of the booth has been its ability to educate through engaging and interactive formats customized to different learning styles," Russo says. "It offered a very personalized experience. The user-friendly technology helped physicians understand the challenges faced by MS patients and how Ampyra may help."
For Acorda, building an educational and interactive exhibit heavy on easily adjusted technology proved to be the right prescription for Ampyra's marketplace debut. And with industry analysts anticipating more than 20 percent of MS patients will eventually use the drug, the diagnosis for Acorda's future couldn't be better. E
|
|
|
|











 t's nearly impossible to avoid the cacophony of pharmaceutical commercials. Just watch prime-time television for an hour or two, and you're likely to see a bevy of pharma ads - many featuring actors skipping through fields of flowers - that all seem to end with a serene voice saying something like "potential side effects include headache, internal bleeding, and nausea." In fact, recent studies estimate the pharmaceutical industry spends between $20 billion and $50 billion a year on marketing, making for a crowded medical marketplace.
t's nearly impossible to avoid the cacophony of pharmaceutical commercials. Just watch prime-time television for an hour or two, and you're likely to see a bevy of pharma ads - many featuring actors skipping through fields of flowers - that all seem to end with a serene voice saying something like "potential side effects include headache, internal bleeding, and nausea." In fact, recent studies estimate the pharmaceutical industry spends between $20 billion and $50 billion a year on marketing, making for a crowded medical marketplace. The second game area featured a round table fitted with 10 iPads. On each, players navigated a neuron field, with touchpoints along the journey highlighting info about the drug. "The iPad interaction was a first-person, 3-D fly-through of a neuron field," Russo says. "Embedded in the 3-D space were points where a fact or video popped up, highlighting where in the neuron field damage may occur due to MS, and show where our drug would potentially interact with that damaged tissue."
The second game area featured a round table fitted with 10 iPads. On each, players navigated a neuron field, with touchpoints along the journey highlighting info about the drug. "The iPad interaction was a first-person, 3-D fly-through of a neuron field," Russo says. "Embedded in the 3-D space were points where a fact or video popped up, highlighting where in the neuron field damage may occur due to MS, and show where our drug would potentially interact with that damaged tissue."


